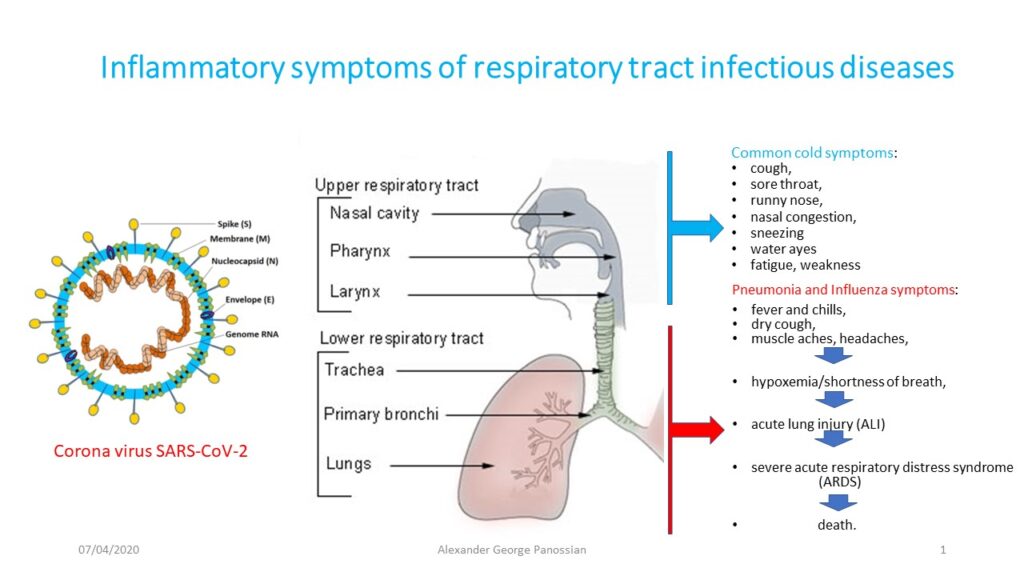
COVID-19
Why coronavirus SARS-CoV-2 is more dangerous than rhinoviruses inducing common cold symptoms?
The viral infections are generally associated with upper respiratory tract infections, of which the signs and symptoms commonly include sneezing, sore throat, cough, runny nose, nasal congestion, water ayes fatigue, weakness; some patients may have lower respiratory tract infections.
In contrast, Severe Acute Respiratory Syndrome Corona Virus SARS-CoV-2 infection may remain asymptomatic in the early stage of CoVDisease-19 (COVID-19) until predominantly infecting the lower respiratory tract and causing severe pneumonia, which sometimes leads to fatal acute lung injury (ALI) and acute respiratory distress syndrome (ARDS), resulting in high morbidity and mortality.
Severe pneumonia caused by SARS-CoV-2 is associated with rapid virus replication, massive inflammatory cell infiltration and elevated proinflammatory cytokine/chemokine responses (“cytokine storm”) resulting in acute lung injury (ALI), and acute respiratory distress syndrome (ARDS).
Mechanism of 2019-nCoV action and therapeutic strategies:
There are two options to combat viral infection by pharmaceutical intervention:
directly affecting virus replication and life cycle
indirectly destroying the virus by regulation of host defense systems including innate and adaptive immune systems, which are deregulated or overcome by virus.
1. Therapeutic intervention directly targeting virus life cycle and replication
A recent publication aimed to identify effective drugs for the treatment of COVID-19 based on systematic analysis of all the proteins encoded by SARS-CoV-2 genes, and target-based virtual ligand screening against compound libraries including ZINC drug database and other databases of natural products reveals that andrographolides (andrographolide, neoandrographolide and 14-deoxy-11,12-didehydro-andrographolide) from Andrographis paniculata, could effectively interact with several targets of SARS-CoV-2 (Wu et al., 2020). Thus, 14-Deoxy-11,12-didehydroandrographolide is a potential inhibitor of RNA-dependent RNA polymerase (RdRp) Nsp12, a conserved protein in coronavirus, which is the vital enzyme of coronavirus replication/transcription complex, while andrographyside – an inhibitor of RdRp and 3C-like main protease (3CLpro), which is essential in the life cycle of the SARS-CoV-2 virus.
It is noteworthy, that several andrographolides have different targets both in the virus (targets preventing the virus RNA synthesis and replication) and in host immune cells (see section below) fighting the virus, that suggest the high therapeutic efficacy of Andrographis.
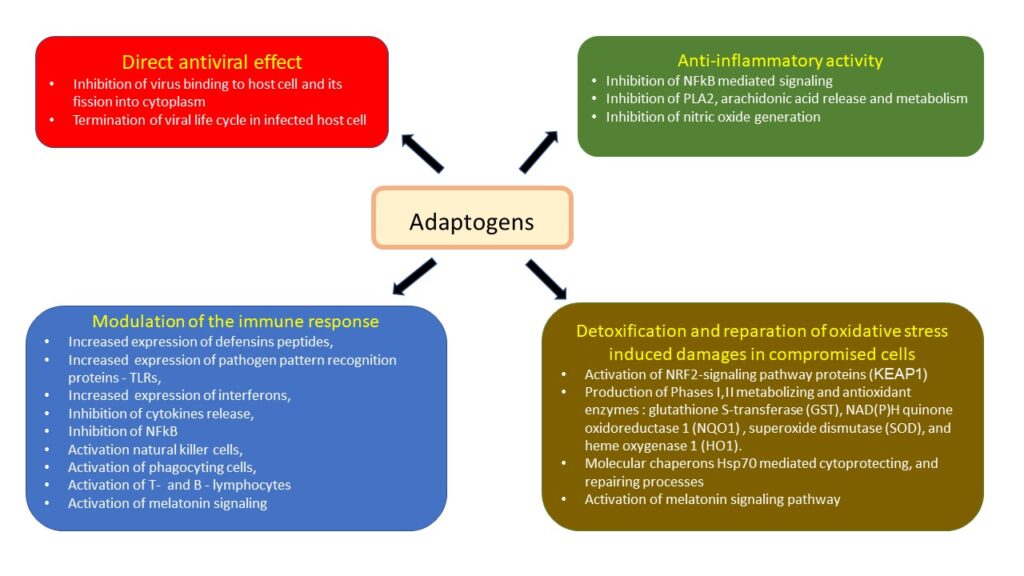
2. Therapeutic intervention via host defense system.
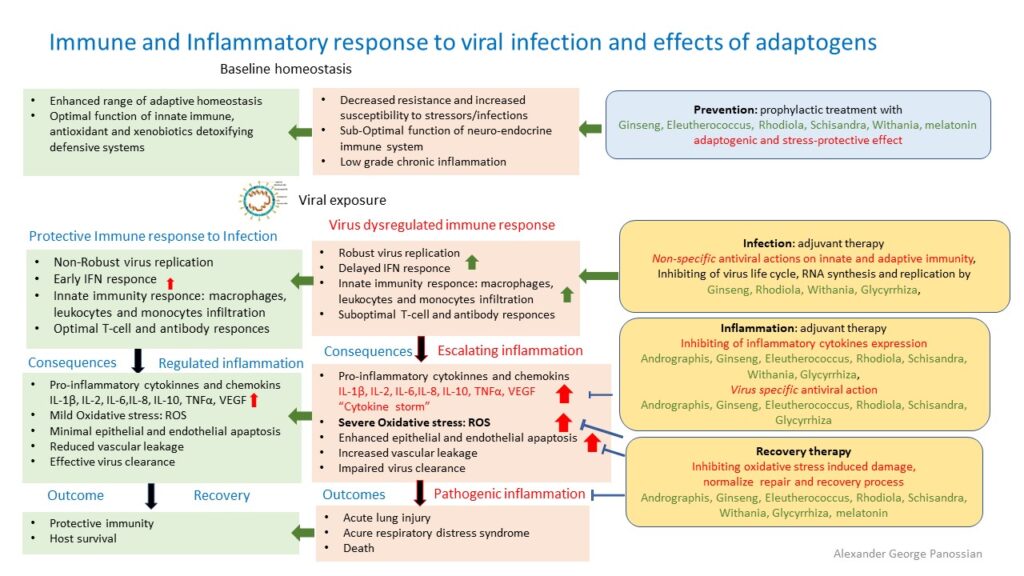
"Good” host protecting, and “bad” host destroying; (pathogenic) inflammatory response of immune system
The inflammatory response is host protecting defense response of immune system aiming to kill infection. Normally, after a virus invades the host, it is first recognized by the host’s innate immune system through pattern recognition receptors (PRRs) including Toll-like receptors (TLR), which then mediate innate immune cell (macrophage) recruitment, eliminates pathogens, ultimately resulting in tissue repair and return to homeostasis (normal healthy balance).
Through different signaling pathways, the virus induces the expression of inflammatory factors, the maturation of dendritic cells, the synthesis of type I interferons (IFNs), and the host’s major antiviral molecules, which limit the spreading of the virus and accelerate macrophage phagocytosis of viral antigens. However, SARSCoV virus is able to escape from the innate immune responses and enter into host cells using similar “keys to the locks of the doors” of the host cells, postpones early IFN-response and replicates robustly.
Next, the adaptive immune response joins the fight against the virus. B lymphocytes produce virus-specific antibodies, while CD8+ T cells directly kill virus-infected cells. T helper cells produce proinflammatory cytokines to help the defending cells. However, CoV can inhibit T cell functions by inducing apoptosis of T cells. The humoral immunity including complements and antibodies are also essential in combating the viral infection.
Coronavirus‐cell interactions lead to the strong production of immune mediators. The secretion of large quantities of chemokines and cytokines, IL‐6, is promoted in infected cells in response to CoV infection. These chemokines and cytokines, in turn, recruit lymphocytes and leukocytes to the site of infection. However, SARS-CoV-2 delayed induces excessive and prolonged cytokine response – “cytokine storms”. This kind of overreaction of the immune system generates a large number of free radicals (“oxidative stress”) that can cause severe damages to the lungs and other organs, and, in the worst scenario, multi-organ failure and even death.
When 2019-nCoV infects the upper and lower respiratory tract it can cause mild or highly acute respiratory syndrome with a consequent release of pro-inflammatory cytokines, including interleukin (IL)-1β and IL-6 (Conti et al, 2020), which are mediators of lung inflammation, fever and fibrosis. Suppression of pro-inflammatory IL-1 family members and IL-6 has been shown to have a therapeutic effect in many inflammatory diseases, including viral infections (Conti et al, 2020). It was concluded that one of the most important mechanism underlying the deterioration of the COVID-19 disease is a cytokine storm (Chen C. et al., 2020), more specifically over-expression of IL-6 (Chen L. et al., 2020). The IL–6 pathway may play an important role in the overactive inflammatory response in the lungs of patients with COVID–19.
Consequences of a cytokine storm and immunopathology and mode of therapeutic action of Andrographis
Therefore, therapeutic interventions targeting these pro-inflammatory cytokines and chemokines could prove beneficial in ameliorating undesirable inflammatory responses
Andrographis and andrographolide inhibits expression of IL-6 and other cytokines
Andrographolide, the main active component of Andrographis paniculata (Burm.f.) Wall. ex Nees, exerts anti-inflammatory effects. Andrographolide dose-dependently inhibited the release and mRNA expression of IL-6, IL-1β and TNF-α, in LPS-stimulated RAW264.7 cells. Andrographolide suppressed LPS-induced NF-κB activation and the phosphorylation of IkBa, ERK1/2, JNK, and p38. These results suggest that Andrographolide exerts an anti-inflammatory effect by inhibiting the activation of NF-κB/MAPK signalling pathway and the induction of proinflammatory cytokines (Li et al., 2016)
Andrographolide significantly inhibited IL-6 and IL-17 in vitro production, suppressed p-Stat3 expression, and inhibited Th17 differentiation of peripheral blood mononuclear cells isolated from 20 Chinese chronic rhinosinusitis with nasal polyps patients and 11 control subjects (Kou et al., 2014).
Andrographis based products are used in many countries for treatment of upper respiratory tract infections. Efficacy of this herbal medicinal product was demonstrated in many clinical and preclinical studies (Panossian et al., 2012). E.g. The administration of AL-1 by oral gavage to mice infected with avian influenza A/Chicken/Guangdong /96 (H9N2), A/Duck/Guangdong/99 (H5N1), and human influenza
A/PR/8/34 (H1N1) viruses greatly reduced the death rate, prolonged life, inhibited lung consolidation,
and reduced viral titers in the lung (Chen et al., 2009)
References
Chen, C; Zhang, XR; Ju, ZY; He, WF. [Advances in the research of cytokine storm mechanism induced by Corona Virus Disease 2019 and the corresponding immunotherapies]. Zhonghua Shao Shang Za Zhi, 2020 vol. 36(0) p. E005
Chen, L; Liu, HG; Liu, W; Liu, J; Liu, K; Shang, J; Deng, Y; Wei, S . [Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi, 2020 vol. 43(3) pp. 203-208
Conti, P; Ronconi, G; Caraffa, A; Gallenga, CE; Ross, R; Frydas, I; Kritas, SK. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents, 2020 vol. 34(2)
Wang, Z; Yang, B; Li, Q; Wen, L; Zhang, R. Clinical Features of 69 Cases with Coronavirus Disease 2019 in Wuhan, China. Clin Infect Dis. 2020 Mar 16. pii: ciaa272. doi: 10.1093/cid/ciaa272.
Gao, Y; Li, T; Han, M; Li, X; Wu, D; Xu, Y; Zhu, Y; Liu, Y; Wang, X; Wang, L. Diagnostic Utility of Clinical Laboratory Data Determinations for Patients with the Severe COVID-19. J. Med. Virol., 2020
Sun, D; Li, H; Lu, XX; Xiao, H; Ren, J; Zhang, FR; Liu, ZS. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center’s observational study. World J Pediatr, 2020
Kou, W; Sun, R; Wei, P; Yao, HB; Zhang, C; Tang, XY; Hong, SL. Andrographolide suppresses IL-6/Stat3 signaling in peripheral blood mononuclear cells from patients with chronic rhinosinusitis with nasal polyps. Inflammation, 2014 vol. 37(5) pp. 1738-43
Li Y, He S, Tang J, Ding N, Chu X, Cheng L, Ding X, Liang T, Feng S, Rahman SU, Wang X, Wu J. Andrographolide Inhibits Inflammatory Cytokines Secretion in LPS-Stimulated RAW264.7 Cells through Suppression of NF-κB/MAPK Signaling Pathway. Evid Based Complement Alternat Med. 2017;2017:8248142. doi: 10.1155/2017/8248142. Epub 2017 Jun 5. PubMed PMID: 28676833; PubMed Central PMCID: PMC5476883.
Chen JX, Xue HJ, Ye WC, Fang BH, Liu YH, Yuan SH, Yu P, Wang YQ. Activity of andrographolide and its derivatives against influenza virus in vivo and in vitro. Biol Pharm Bull. 2009 Aug;32(8):1385-91. PubMed PMID: 19652378.
Panossian A. Wikman G. Efficacy of Andrographis paniculata in upper respiratory tract (URT) infectious diseases and the mechanism of action. In: Evidence and rational based research on Chinese Drugs, Ed. H. Wagner and G. Ulrich Merzenich (Eds.), Springer Publ. Comp. 2012. Pp. 137-180.
Panossian A, Seo EJ, Wikman G, Efferth T. Synergy assessment of fixed combinations of Herba Andrographidis and Radix Eleutherococci extracts by transcriptome-wide microarray profiling. Phytomedicine. 2015 Oct 15;22(11):981-92.
Wu C et al., 2020. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharmaceutica Sinica B Available online 27 February 2020 In Press, Journal Pre-proof, https://www.sciencedirect.com/science/article/pii/S2211383520302999#!
Panossian A, Brendler T. The Role of Adaptogens in Prophylaxis and Treatment of Viral Respiratory Infections. Pharmaceuticals (Basel). 2020 Sep 8;13(9):E236. doi:10.3390/ph13090236. PMID: 32911682. https://www.mdpi.com/1424-8247/13/9/236
Brendler, T., Al-Harrasi, A., Bauer, R., Gafner, S., Hardy, M. L., Heinrich, M., Hosseinzadeh, H., Izzo, A. A., Michaelis, M., Nassiri-Asl, M., Panossian, A., Wasser, S. P., & Williamson, E. M. (2021). Botanical drugs and supplements affecting the immune response in the time of COVID-19: Implications for research and clinical practice. Phytotherapy research : PTR, 35(6), 3013–3031. https://doi.org/10.1002/ptr.7008
Karosanidze I, Kiladze U, Kirtadze N, Giorgadze M, Amashukeli N, Parulava N, Iluridze N, Kikabidze N, Gudavadze N, Gelashvili L, Koberidze V, Gigashvili E, Jajanidze N, Latsabidze N, Mamageishvili N, Shengelia R, Hovhannisyan A, and Panossian A. Efficacy of Adaptogens in Patients with Long COVID-19: A Randomized, Quadruple-Blind, Placebo-Controlled Trial. Pharmaceuticals 2022, 15(3), 345; https://doi.org/10.3390/ph15030345