Adaptogens are natural stress-protective compounds or plant extracts that increase the adaptability, resilience, and survival of organisms. The first definition of adaptogens is dated 1958. Tremendous progress in biology and medicine during the last 60 years has had a significant impact on the research of adaptogens. Consequently, a better understanding of how they work in the organisms resulted in the evolution of the definition of adaptogens.
The milestones of adaptogens research and use in medicine: Monograph
Adaptogens book: TTN publishing 2022
Year Definition
1958 Adaptogens are compounds that increase the “state of non-specific resistance” in stress (Lazarev, 1958).
1968 Adaptogens are innocuous agents, nonspecifically increasing resistance against physically, chemically, biologically, and psychologically noxious factors (“stressors”), normalizing effect independent of the nature of pathologic state (Brekhman, 1968).
1984 The adaptogens are nontoxic compounds with polyvalent mechanisms of action and pharmacological effects related to adaptability and survival. (Farnsworth et al., 1984)
1994 Adaptogens are substances which elicit in an organism a state of nonspecifically raised resistance, allowing them to counteract stressor signals and to adapt to exceptional strain (Wagner et al., 1994).
1999 Plant adaptogens are agents which reduce damaging effects of various stressors due to reduction of the reactivity of host defense system. They adapt organism to stress and have curative effect in stress induced disorders (Panossian et al., 1999).
1999 Adaptogens are metabolic regulators, which increase the ability of an organism to adapt to environmental factors and to avoid damage from such factors (Panossian et al., 1999).
2007 Adaptogenic substances have the capacity to normalize body functions and strengthen systems compromised by stress. They have a protective effect on health against a wide variety of environmental assaults and emotional conditions (EMEA/HMPC/102655/2007).
2009 Adaptogens comprise a pharmacotherapeutic group of herbal preparations used to: increase attention and endurance in fatigue and prevent/mitigate/reduce stress-induced impairments and disorders related to neuro-endocrine and immune systems [Panossian and Wikman, 2009].
2017 Botanical adaptogens are plant extracts, or specific constituents of plant extracts, which function to increase survival in animals and humans by stimulating their adaptability to stress by inducing adaptive responses (Panossian and Amsterdam, 2017).
2017 Adaptogens are stress-response modifiers that increase an organism’s nonspecific resistance to stress by increasing its ability to adapt and survive. (Panossian, 2017).
2017 Botanical adaptogens are metabolic regulators that increase survival by increasing adaptability in stress. (Panossian, 2017).
2018 Adaptogens are natural compounds or plant extracts that increase adaptability and survival of living organisms to stress (Panossian et al., 2018).
??? Adaptogen – any of various natural substances used in herbal medicine to normalize and regulate the systems of the body. https://www.dictionary.com/browse/adaptogen.

Chemical class
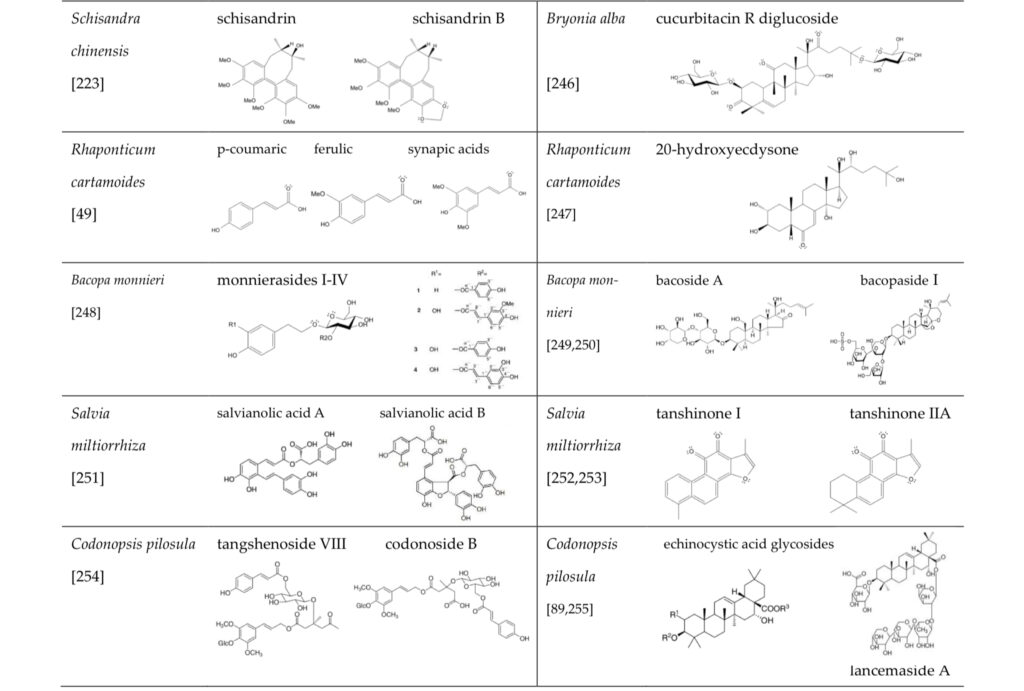
The principal active constituents of adaptogenic plants (as investigated thus far, table 1) can be divided into two main chemical groups (1):
- Terpenoids, with a tetracyclic skeleton such as cortisol and testosterone: ginsenosides, sitoindosides, cucurbitacines, and withanolides.
- Aromatic compounds, that are structurally like catecholamines or tyrosine, including:
- lignans: e.g. eleutheroside E (Eleutherococcus senticosus), schizandrin B (Schizandra chinensis)
- phenylpropane derivatives: e.g. syringin (Eleutherococcus senticosus), rosavin (Rhodiola rosea)
- phenylethane derivatives: e.g. salidroside, tyrosol (Rhodiola rosea).
Many studies indicate direct interactions between tetracyclic terpenoids and corticosteroid and estrogenic receptors (1).

Botanical origin
Table 1. Plants Mentioned in Literature as Adaptogens (1, 98)*
Ajuga turkestanica (Regel) Briq.
Alstonia scholaris (L.) R. Br.
Alstonia scholaris (L.) R. Br.
Andrographis paniculata(Burm.f.) Nees (92)
Aralia mandshurica Rupr. & Maxim
Argyreia nervosa (Burm. f.) Bojer
Argyreia speciosa (L. f.) Sweet
Asparagus racemosus Wild
Bacopa monnieri (L.) Wettst
Bergenia crassifolia (L.) Fritsch
Bryonia alba L.
Caesalpinia bonduc (L.) Roxb
Centella asiatica (L.) Urb.
Chlorophytum borivilianum Santapau & R.R.Fern.
Chrysactinia mexicana A. Gray
Cicer arietinum L.
Codonopsis pilosula (Franch.) Nannf.
Convolvulus prostratus Forssk.
Curculigo orchioides Gaertn.
Curcumin from Turmeric (Curcuma longa)
Dioscorea deltoidea Wall. ex Griseb.
Dioscorea roxburghii (Wall.) Hurus.
Echinopanax elatus Nakai
Eleutherococcus senticosus (Rupr. & Maxim.) Maxim.
Emblica officinalis Gaetrn.
Eucommia ulmoides Oliv.
Evolvulus alsinoides (L.) L.
Firmiana simplex (L.) W.Wight
Gentiana pedicellata (D.Don) Wall
Glycyrrhiza glabra L.
Heteropterys aphrodisiaca Machado
Hippophae rhamnoides L.
Holoptelea integrifolia Planch
Hoppea dichotoma Willd.
Hypericum perforatum L.
Lepidium peruvianum/ Lepidium meyenii Walp.
Ligusticum striatum DC.
Melilotus officinalis (L.) Pall.
Morus alba L.
Mucuna pruriens (L.) DC.
Nelumbo nucifera Gaertn.
Ocimum sanctum L.
Oplopanax elatus (Nakai) Nakai
Panax ginseng C.A.Meyer.
Panax pseudoginseng Wall.
Pandanus odoratissimus L.f.
Paullinia cupana Kunth
Pfaffia paniculata (Mart.) Kuntze
Piper longum L.
Potentilla alba L.
Ptychopetalum olacoides Benth.
Rhaponticum carthamoides (Willd.) Iljin
Rhodiola heterodonta (Hook. f. & Thomson) Boriss.
Rhodiola rosea L.
Rostellularia diffusa (Willd.) Nees.
Salvia miltiorrhiza Bunge
Schisandra chinensis (Turcz.) Baill.
Scutellaria baicalensis Georgi
Serratula inermis
Sida cordifolia L.
Silene italica (L.) Pers.
Sinomenium acutum (Thunb.) Rehder & E.H.Wilson
Solanum torvum SW.
Sutherlandia frutescens (L.) R.Br.
Terminalia chebula Retz.
Tinospora cordifolia (Willd.) Miers
Trichilia catigua A.Juss.
Trichopus zeylanicus Gaertn.
Turnera diffusa Willd. ex Schult.
Vitis vinifera L.
Withania somnifera (L.) Dunal
*This table is an update from the reviews Wagner et al. 1994 and Panossian and Wagner 2011. It includes plants which do and do not meet the formal definition of adaptogen. In various countries, adaptogens are used as dietary supplements or/and conventional and traditional medicinal products (2-5).
Therapeutic category/pharmacological group: adaptogens
Pharmacological activity: stress-protective, stimulating, tonic
Mechanism of action: multitarget effect on neuroendocrine-immune system including:
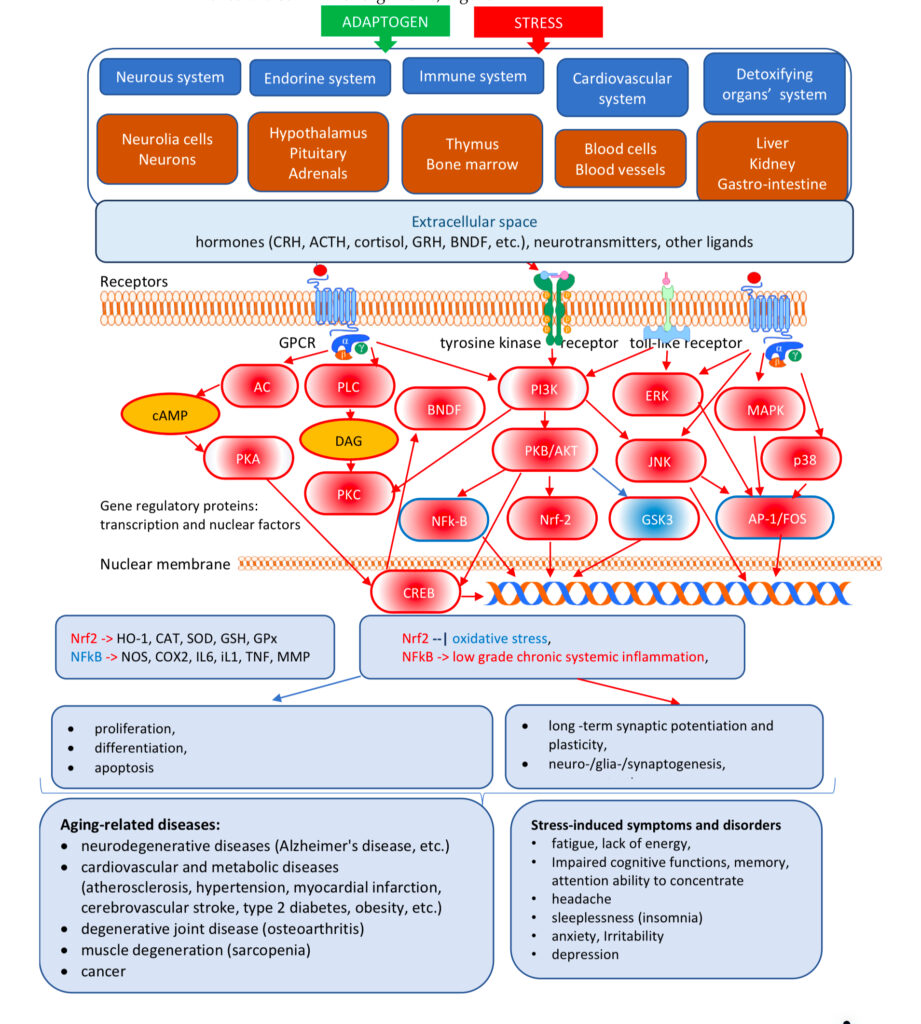
triggering of intracellular and extracellular adaptive signaling pathways that promote cell survival and organismal resilience in stress
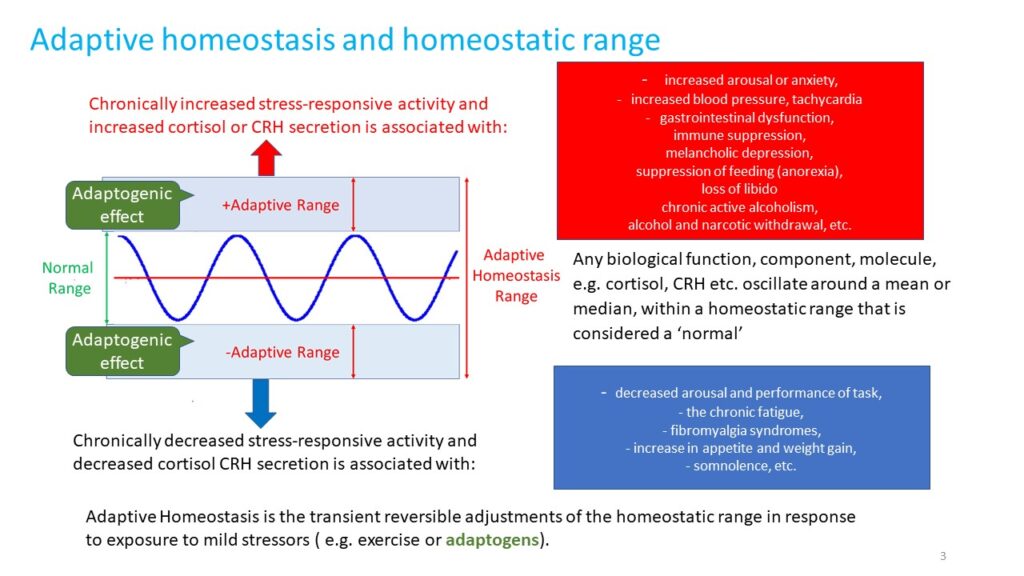
regulation of metabolism and homeostasis via effects on expression of stress hormones (corticotropin and gonadotropin releasing hormones, urocortin, cortisol, neuropeptide Y, heat shock proteins Hsp70) and their receptors
Indications/health claims: stress-induced fatigue, mental and behavioral disorders, aging associated disorders, infectious diseases .
What is necessary and sufficient to be considered as an adaptogenic plant?
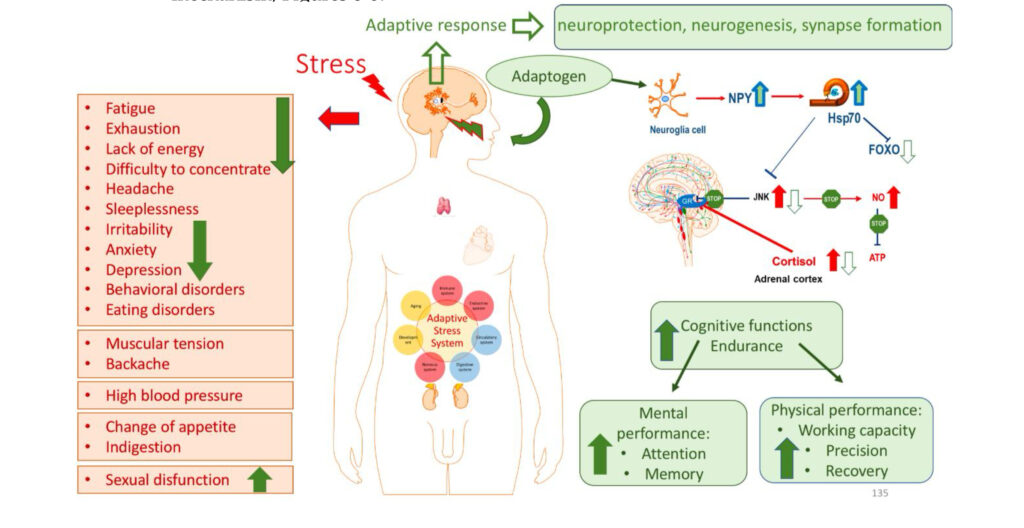
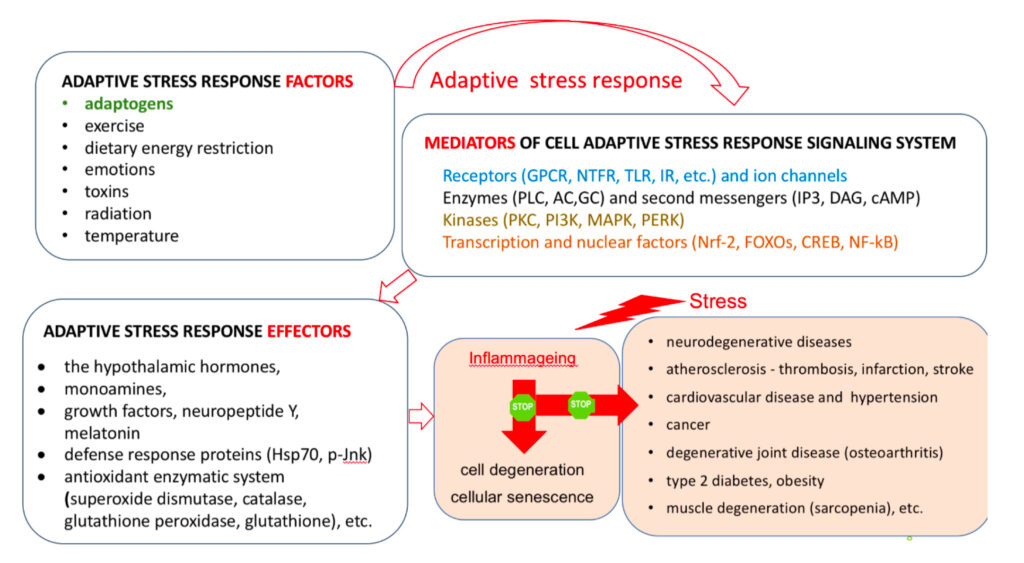
An adaptogen activates cellular adaptive signaling pathways (e.g. MAPK and I3PK mediated pathways) that are known promotes survival in response to stress and suggests neuroprotective activity and potential benefits of adaptogens in neurodegenerative diseases. In addition to activating of multiple cytoprotecting mechanisms increasing cell survival (antioxidant, immune modulation, Hsp70 modulation), adaptogens trigger generation of hormones (e.g. CRH, GRH, urocortin, cortisol), playing key role in metabolic regulation and homeostasis. Therefore, adaptogens are active in numerous conditions and diseases associated with stress and aging related impairment of neuroendocrine – immune complex, energy, fatigue, etc. Overall, the mechanisms of action of adaptogens are “specifically” related to stress-protective activity and increased adaptability of the organism (1, 98).

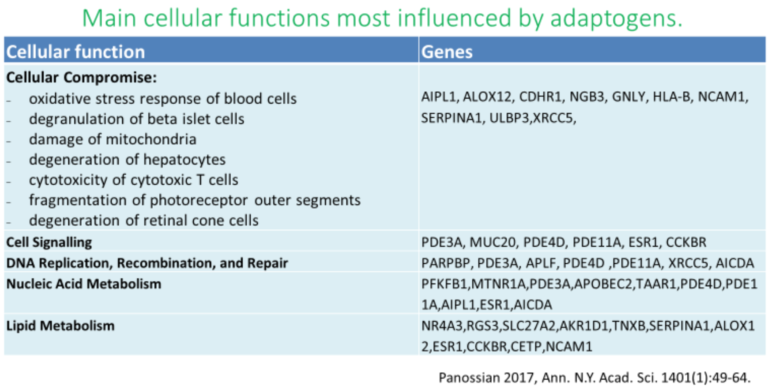
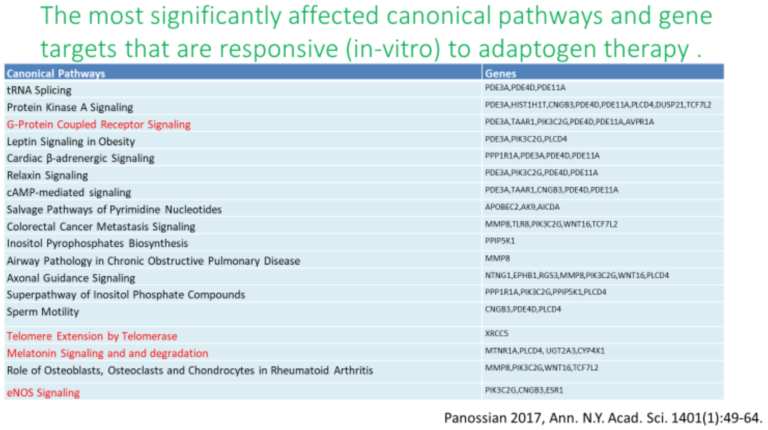
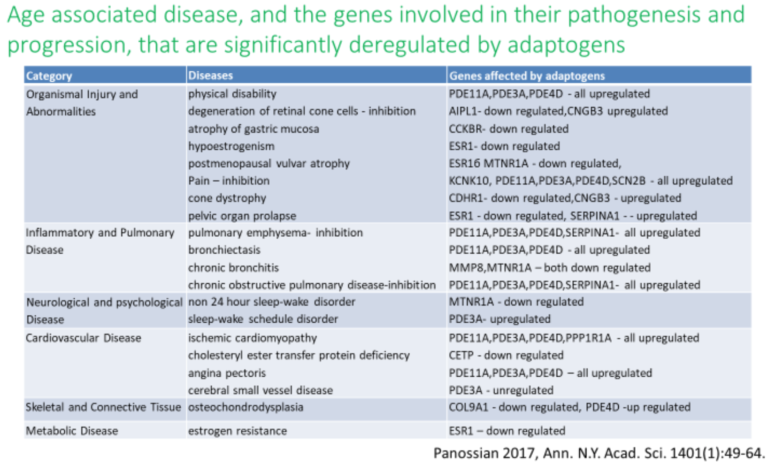
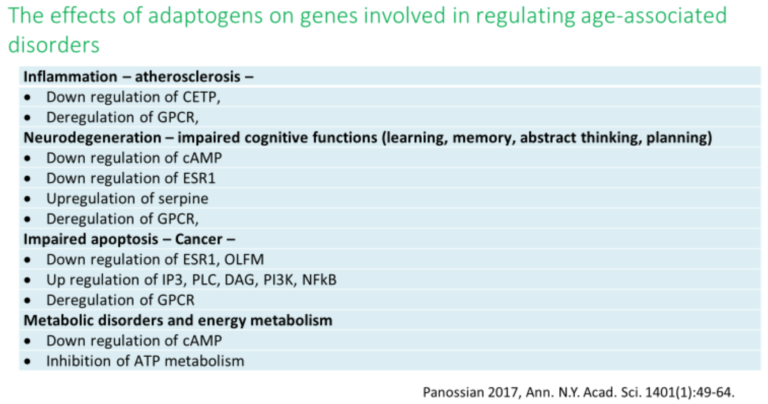
History and Background
The term adaptogens is used in alternative and complimentary medicine, pharmacognosy, phytomedicine, phytopharmacology, and phytotherapy research (6-14). Initially, the term adaptogen was coined to describe substances that can increase the “state of non – specific resistance to stress” (15,16). Originally, the adaptogenic concept (3,7-12) was based on Hans Selye’s theory (17,18) of adaptive stress response of neuroendocrine immune complex (19,20) and long-term traditional use of some medicinal plants that are believed to promote physical and mental health, improve defense mechanisms of the body, and enhance longevity (7-14, 21-33). It was suggested that certain compounds and herbal extracts, termed adaptogens, could diminish the magnitude of the alarm phase of adaptive stress response and prolong the duration the phase of non-specific resistance to stress (7,15). Based on evidences mainly from animal studies, adaptogens were defined as nontoxic “metabolic regulators, which increase the ability of an organism to adapt to environmental factors and to avoid damage from such factors” (6, 9). It should be emphasized that the term “adaptogen” is associated with a physiological process – adaptation to environmental challenges, which is a multistep process that involves diverse mechanisms of intra- and extracellular interactions. The updated definition of adaptogens (1) is supported by results of recent studies of molecular mechanisms of action of adaptogens on a variety of regulatory systems from the cellular level to whole organism (1, 34-48).
- Panossian A. Adaptogens: A Historical Overview and Perspective. NATURAL PHARMACY, 2003, July/August 2003, Vol.7,No.4, pp. 1-5. http://www.scicompdf.se/adaptogener/panossian_2.pdf
- Panossian A, Wagner H. Adaptogens: A Review of their History, Biological Activity, and Clinical Benefits. HerbalGram 90 Page: 52-63 ; https://www.herbalgram.org/resources/herbalgram/issues/90/table-of-contents/feat_adaptogens/ ; https://www.herbalgram.org/resources/herbalgram/issues/90/
Pharmacology and the Mechanism of Action
There are many molecular targets for stress response modifiers since stress response and adaptation to environmental challenge are multistep processes that involve intracellular and extracellular signaling pathways at all levels of stress regulation.
Stimulating and stress-protective effects are characteristic and common pharmacological effects of adaptogens (9,11,31). These have been observed in many animals and humans by testing their cognitive function and physical endurance under stressful conditions (8,9,11,39, 42,43). The main difference between adaptogens and conventional stimulants, such as caffeine, amphetamine, etc., is that after prolonged use, the later can cause the user to develop both tolerance and addiction, Table 2 (12, 43). Adaptogens exhibit polyvalent beneficial effects against chronic inflammation, atherosclerosis, neurodegenerative cognitive impairment, metabolic disorders, cancer, and other aging-related diseases (22, 27, 49, 50). All of them are associated with the metabolic regulation of homeostasis and threatened adaptability of stress system.
Table 2. The Differences in Properties Between Adaptogens and Other Stimulants.
| Stimulants | Adaptogens | |
Stress protective (neuro-, hepato-, cardio-protective) | No | High |
Recovery process after exhaustive physical load | Low | High |
Energy depletion | Yes | No |
Performance in stress | – | Increased |
Survival in stress | – | Increased |
Quality of arousal | Poor | Good |
Addiction potential | Yes | No |
Side effects | Yes | Rare |
DNA/proteins synthesis | Decreased | Increased |
NPY mediated activation of Hsp70 | – | Increased |
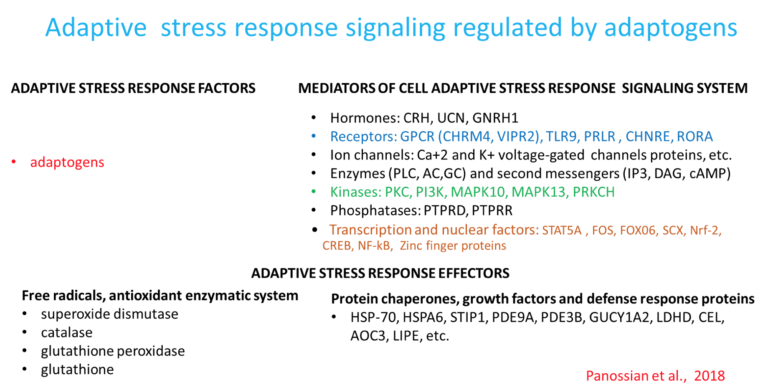
The metabolic regulation of homeostasis by adaptogens at the cellular and systemic level is associated with multiple targets (1, 34-47). Consequently, the pharmacology of adaptogens is a typical example of network pharmacology that can be approached using the systems biology concept (1). The classic reductionist model that presumes a specific receptor/drug interaction (51) is unsuitable for this scenario and insufficient when attempting to understand the mechanism of action of adaptogens. Molecular targets, signaling pathways, and networks common to adaptogens have been identified (1, 34-47). They are associated with stress hormones and key mediators of the regulation of homeostasis (molecular chaperons Hsp70, neuropeptide Y, G protein-coupled receptors, dopamine-cAMP-PKA-CERT, IP3, PLC, DAG, PI3K, NFkB, mediated signaling pathways, stress activated kinase JNK, FOXO3, cortisol, estrogens, nitric oxide, etc.) (1, 34-47).
Adaptogens exert a polyvalent biological activity and provoke multiple effects at the transcriptional level of regulation of cellular metabolism and homeostasis. The genome-wide effects of several adaptogenic herbal extracts in brain cells culture were recently elucidated (34-36, 89-94). These data highlight the consistent activation of adaptive stress response signaling pathways (ASRSPs) by adaptogens in T98G neuroglia cells. The extracts affected many genes playing key roles in modulation of adaptive homeostasis, indicating their ability to modify gene expression to prevent stress-induced and aging-related disorders. At least 88 of the 3516 genes regulated by various adaptogens in isolated brain cells were closely associated ASRSPs, including neuronal signaling related to corticotropin-releasing hormone, cAMP-mediated, protein kinase A, and CREB; pathways related to signaling involving CXCR4, melatonin, nitric oxide synthase, GP6, Gαs, MAPK, neuroinflammation, neuropathic pain, opioids, renin–angiotensin, AMPK, calcium, and synapses; and pathways associated with dendritic cell maturation and G-coupled protein receptor–mediated nutrient sensing in enteroendocrine cells (90). All adaptogens tested showed significant effects on the expression of genes encoding neurohormones CRH, GNRH, UCN, G-protein coupled and other transmembrane receptors TLR9, PRLR, CHRNE, GP1BA, PLXNA4, a ligand-dependent nuclear receptor RORA, transmembrane channels, transcription regulators FOS, FOXO6, SCX, STAT5A, ZFPM2, ZNF396, ZNF467, protein kinases MAPK10, MAPK13, MERTK, FLT1, PRKCH, ROS1, TTN), phosphatases PTPRD, PTPRR, peptidases, metabolic enzymes, a chaperone (HSPA6), and other proteins, all of which modulate numerous life processes, playing key roles in several canonical pathways involved in defense response and regulation of homeostasis in organisms (90).
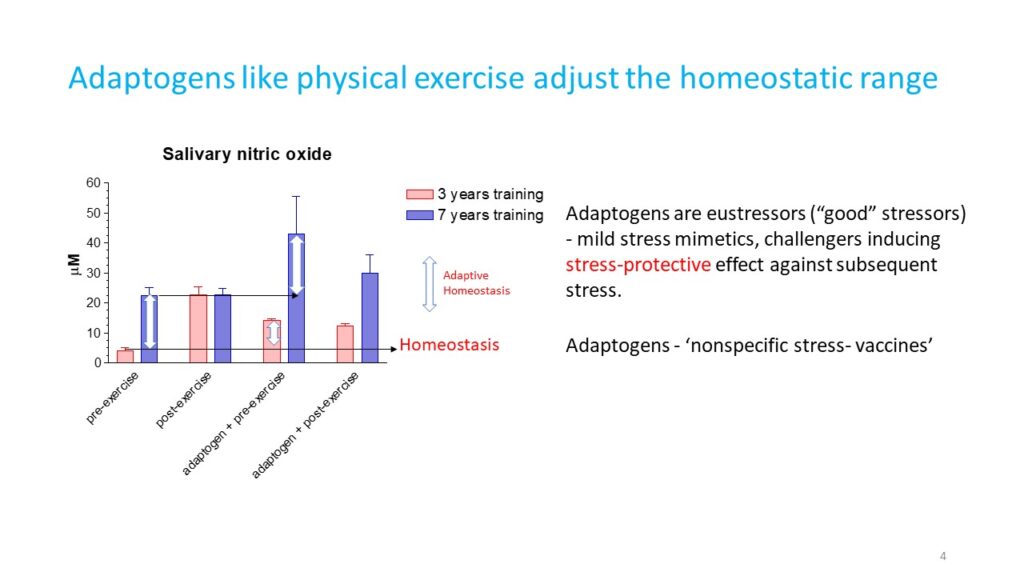
A characteristic feature of adaptogens is that they act as eustressors (i.e. “good stressors”), and as mild stress mimetics or ‘stress- vaccines’ that induce a stress-protective response (44, 47). Adaptogens exhibit multitarget action and the shared use of several different receptors, including receptors for corticosteroid, mineralocorticoid, progestin, estrogen, serotonin, NMDA, and nicotinic acetylcholine, receptor tyrosine kinases, and many G-protein coupled receptors (1, 34-47, 50,52-72). Therefore, the possibility that numerous molecular network interactions (with feedback regulation of the neuroendocrine and immune systems) contribute to the overall pharmacological response and result in agonist-dependent antagonism is most suitable for understanding the mechanisms of action of adaptogens (1).
Molecular targets, signaling pathways, and networks common to adaptogens are associated with chronic inflammation, atherosclerosis, neurodegenerative cognitive impairment, metabolic disorders, and cancer, all of which are more common with age (14, 34,35,36).
Current and Potential Use
Current and potential uses of adaptogens are mainly related to stress-induced fatigue and cognitive function, mental illness, and behavioral disorders, infectious diseases (9, 13, 32, 42, 43, 76-85, 89-100). Their prophylactic use by healthy subjects to ameliorate stress and prevent age-related diseases appears to be justified (14, 21, 23-26, 42,43, 89-93), e.g. Rhodiola rosea, Withania somnifera and Eleutherococcus senticosus downregulate the expression of key genes (ALOX5AP, DPEP2, LTC4S) involved biosynthesis of leukotrienes A, B, C, D and E, resulting in inhibition of leukotriene signaling pathway suggesting their potential benefits in Alzheimer disease (89). Recent observations indicate on potential beneficial effects of adaptogens on neuronal functions associated with mild cognitive impairments in cancer chemotherapy (91-93, 95-97).
In a number of clinical studies, beneficial effects of adaptogens have been demonstrated on healthy subjects in stress conditions (42,43, 73-81, 95-97). This is especially true of the mental and physical performance of fatigue and mental strain (42,43, 73-81, 95-97). Furthermore, efficacy of adaptogens in mild and moderate depression has been demonstrated (32, 82-85).
Adaptogens have been used for the last decades in the official medicine of Russia and neighbor countries (Estonia, Ukraine, Armenia, Kazakhstan, etc.) as a stimulant against fatigue by patients who suffered asthenic states and by healthy people who showed asthenia during periods of high mental exertion or after intensive physical work (2, 27, 86, 87). Adaptogens are also applied in psychiatric practice. (13, 32, 84, 85).
In Sweden, Norway and Denmark, rhodiola traditional herbal medicinal product is indicated as an adaptogen in situations of decreased performance such as fatigue and sensation of weakness.
Dietary supplements containing rhodiola, withania, ginseng, eleutherococcus, schisandra and other adaptogenic plant extracts are widely used all over the world (6).
References
- Panossian AG, 2017. Understanding adaptogenic activity: specificity of the pharmacological action of adaptogens and other phytochemicals. Ann NY Acad Sci. 22 JUN 2017
http://onlinelibrary.wiley.com/doi/10.1111/nyas.13399/full
http://onlinelibrary.wiley.com/doi/10.1111/nyas.13399/pdf - Shikov AN, Pozharitskaya ON, Makarov VG, Wagner H, Verpoorte R, Heinrich M. 2014. Medicinal plants of the Russian Pharmacopoeia; their history and applications. J Ethnopharmacol. 154(3):481-536.
- Reflection Paper on the Adaptogenic Concept. European Medicines Agency Evaluation of Medicines for Human Use. London, 8 May 2008. Doc. Ref. EMEA/HMPC/102655/2007. www.ema.europa.eu/docs/en_GB/document…/WC500003646.pdf
- FDA (Food and Drug Administration), USA. Notice of proposed rule marketing. Federal Register, Anon, April 29, 1998.
- EFSA Consolidated List of Article 13 Health Claims of the European Food Safety Authority (EFSA). Legal and regulatory framework for herbal medicines. Association of the European Self-Medication Industry (AESMI). Brussels, April 2010. P.151-158.
- Samuelsson G, Bohlin L. 2009. Drugs of Natural Origin: A Treatise of Pharmacognosy, 6 ed. Swedish Academy of Pharmaceutical Sciences, Stockholm, Sweden.
- Brekhman II, Dardymov IV. 1968. New substances of plant origin increase nonspecific resistance. Annu Rev Pharmacol. 8: 419-430.
- Wagner H, Nörr H, Winterhoff H. 1994. Plant adaptogens. Phytomedicine 1: 63-76.
- Panossian A.G. 2003. Adaptogens: Tonic herbs for fatigue and stress. Alternative & Complementary Therapies 9: 327-332.
- Panossian A, Wikman G, Wagner H. 1999. Plant adaptogens. III. Earlier and more recent aspects and concepts on their mode of action. Phytomedicine 6: 287-300.
- Panossian, A., Wagner H. 2005. Stimulating effect of adaptogens: an overview with particular reference to their efficacy following single-dose administration. Phytother Res 19: 819-838.
- Panossian A, Wagner H. 2011. Adaptogens. A Review of their History, Biological Activity, and Clinical Benefits. HerbalGram 90: 52-63.
- Panossian AG. 2013. Adaptogens in Mental and Behavioral Disorders. Psychiatr Clin North Am. 36: 49-64.
- Panossian A, Gerbarg P. 2016. “Potential Use of Plant Adaptogens in Age-related Disorders.” Complimentary, Alternative, and Integrative Interventions in Mental Health and Aging. H. Lavretsky, M. Sajatovic & C.F. Reynolds III, Eds.: 197-211. New York: Oxford University Press.
- Lazarev NV. 1958. General and specific in the action of pharmacological agents. Farmacol. Toxicol. 21: 81-86.
- Lazarev NV, Ljublina EI, Ljublina MA. 1959. State of nonspecific resistance. Patol Fiziol ExperimTerapia. 3: 16-21.
- Selye H. 1976. Forty years of stress research: principal remaining problems and misconceptions. Can Med Assoc J. 115: 53-56.
- Selye H. 1938. Experimental evidence supporting the conception of “adaptation energy” Am J Physiol. 123: 758–765.
- Chrousos GP, Gold PW. 1992. The concept of stress system disorders: Overview of behavioral and physical homeostasis. JAMA. 267:1244-1252.
- Stratakis CA, Chrousos GP. 1995. Neuroendocrinology and pathophysiology of the stress system. Ann N Y Acad Sci. 771, 1-18.
- Panossian A, Wikman G. 2014. “Evidence based efficacy and effectiveness of Rhodiola SHR-5 extract in treating stress- and age-associated disorders”. Chapter 9 In the book Rhodiola rosea. Cuerrier. A & K. Ampong-Nyarko, Eds. Series: Traditional Herbal Medicines for Modern Times, CRC Press, pp. 203-221.
- Panossian A, Wikman G. 2008. Pharmacology of Schisandra chinensis Bail.: an overview of Russian research and uses in medicine. J Ethnopharmacol. , 118(2):183-212.
- Kumar R, Gupta K, Saharia K, et al. 2013. Withania somnifera root extract extends lifespan of Caenorhabditis elegans. Ann Neurosci. 20:13-16.
- Jafari M, Felgner JS, Bussel II, et al.2007. Rhodiola: a promising anti-aging Chinese herb. Rejuvenation Res. 10:587-602.
- Lee JH, Choi SH, Kwon OS, et al. 2007. Effects of ginsenosides, active ingredients of Panax ginseng, on development, growth, and life span of Caenorhabditis elegans. Biol Pharm Bull. 30: 2126-2134.
- Wiegant FA, Surinova S, Ytsma E, et al. 2009. Plant adaptogens increase lifespan and stress resistance in C. elegans. Biogerontology. 10: 27–42.
- Panossian A, Wikman G, Sarris J. 2010. Rosenroot (Rhodiola rosea): traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine. 7:481-93.
- Patel S, Rauf A. 2017. Adaptogenic herb ginseng (Panax) as medical food: Status quo and future prospects. Biomed Pharmacother. 85:120-127.
- Rege NN, Thatte UM, Dahanukar SA. Adaptogenic properties of six rasayana herbs used in Ayurvedic medicine. Phytother Res. 1999 Jun;13(4):275-91.
- Singh N, Bhalla M, de Jager P, Gilca M. An overview on ashwagandha: a Rasayana (rejuvenator) of Ayurveda. Afr J Tradit Complement Altern Med. 2011;8(5 Suppl):208-13. doi: 10.4314/ajtcam.v8i5S.9. Epub 2011 Jul 3.
- Boon-Niermeijer EK, van den Berg A, Vorontsova ON, et al. 2012. Enhancement of Adaptive Resistance against a Variety of Chronic Stress Conditions by Plant Adaptogens: Protective Effects on Survival and Embryonic Development of Lymnaea stagnalis. Adaptive Medicine 4: 233-244.
- Amsterdam JD, Panossian AG. 2016. Rhodiola rosea L. as a putative botanical antidepressant. Phytomedicine 23:770-783
- Farnsworth NR, Waller D, Strelkoffa LM. 1984. “Use of Eleutherococcus senticosus in Unuted States: problems, prospects and literature update”. In: New data on Eleutherococcus. Proceedings of the Second International Symposium on Eleutherococcus. Brekhman, I.I., I.V.Dardimov, S.E. Lee, et al., Eds.: 47-51., Vladivostok: Far East Sci.Center, USSR Acad.Sci. Moscow, 1986.
- Panossian A, Hamm, Kadioglu R, Wikman O, Efferth T. 2014. Mechanism of action of Rhodiola, salidroside, tyrosol and triandrin in isolated neuroglial cells: an interactive pathway analysis of the downstream effects using RNA microarray data. Phytomedicine 21: 1325–1348. https://www.academia.edu/16759912/Mechanism_of_action_of_Rhodiola_salidroside_tyrosol_and_triandrin_in_isolated_neuroglial_cells_an_interactive_pathway_analysis_of_the_downstream_effects_using_RNA_microarray_data
- Panossian A, Hamm R, Wikman G, Kadioglu O, Efferth T. 2013. Synergy and antagonism of active constituents of ADAPT-232 on transcriptional level of metabolic regulation in isolated neuroglia cells. Front Neurosci. 20:7:16. http://www.frontiersin.org/Journal/Abstract.aspx?s=744&name=neuroendocrine_science&ART_DOI=10.3389/fnins.2013.00016
- Panossian A, Seo EJ, Wikman G, Efferth T. 2015. Synergy assessment of fixed combinations of Herba Andrographidis and Radix Eleutherococci extracts by transcriptome-wide microarray profiling. Phytomedicine. 22: 981-992.
https://www.sciencedirect.com/science/article/pii/S0944711315002408?via%3Dihub - Panossian A, Wikman G, Kaur P, Asea A. 2009. “Adaptogens exert a stress-protective effect by modulation of expression of molecular chaperones”. Phytomedicine 16 (6-7): 617-622. https://www.sciencedirect.com/science/article/pii/S0944711308002250?via%3Dihub
- Panossian A, Wikman G, Kaur, P, Asea, A. (2010). “Molecular Chaperones as Mediators of Stress Protective Effect of Plant Adaptogens”. Heat Shock Proteins and Whole Body Physiology. Heat Shock Proteins. 5. Jumps. pp. 351-364
- Panossian AG, Wikman G, Kaur P, Asea A. (2012). “Adaptogens Stimulate Neuropeptide Y and Hsp72 Expression and Release in Neuroglia Cells”. Frontiers in Neuroscience 6: 6. http://www.frontiersin.org/neuroendocrine_science/10.3389/fnins.2012.00006/full
- Asea A. Kaur P, Panossian A, Wikman G. 2013. Evaluation of molecular chaperons Hsp72 and neuropeptide Y as characteristic markers of adaptogenic activity of plant extracts. Phytomedicine. 20:1323-1329. https://www.sciencedirect.com/science/article/pii/S0944711313002456?via%3Dihub
- Panossian A, Hambartsumyan M, Hovanissian A, Gabrielyan E, and Wilkman G. (2007). The Adaptogens Rhodiola and Schizandra Modify the Response to Immobilization Stress in Rabbits by Suppressing the Increase of Phosphorylated Stress-Activated Protein Kinase, Nitric Oxide and Cortisol. Drug Targets Insights 1, 39-54. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3155223/
- Panossian A, Wikman G. (2009). Evidence-based efficacy of adaptogens in fatigue, and molecular mechanisms related to their stress-protective activity. Current Clinical Pharmacology 4 (3): 198-219. Archived from the original on May 10, 2013.
https://www.academia.edu/3827272/Evidence-Based_Efficacy_of_Adaptogens_in_Fatigue_and_Molecular_Mechanisms_Related_to_their_Stress-Protective_Activity - Panossian A, Wikman G. 2010. Effects of Adaptogens on the Central Nervous System and the Molecular Mechanisms Associated with Their Stress—Protective Activity. Pharmaceuticals 3: 188-224.
- Panossian A, Gabrielian E, Wagner H. 1999. On the mechanism of action of plant adaptogens with particular reference to cucurbitacin R diglucoside. Phytomedicine 6:147-155.
- Mohanan P, Subramaniyam S, Mathiyalagan R, et al. 2017. Molecular signaling of ginsenosides Rb1, Rg1, and Rg3 and their mode of actions. Journal of Ginseng Research. In Press, Uncorrected Proof, Available online 19 January 2017
- Qi HY, Li L, Ma H. Cellular stress response mechanisms as therapeutic targets of ginsenosides. Med Res Rev. 2017 Jun 6.
- Wiegant FAC, Limandjaja G, de Poot SAH, et al. 2008. “Plant adaptogens activate cellular adaptive mechanisms by causing mild damage”. In: Adaptation Biology and Medicine: Health Potentials. Lukyanova, L., N. Takeda & P.K. Singal, Eds. NewDelhi, India, 2008; Volume 5. pp. 319–332. Narosa Publishers.
- Stranahan AM, Mattson MP. 2012. Recruiting adaptive cellular stress responses for successful brain ageing. Nat Rev Neurosci. 13: 209-216.
- Khanum F, Bawa AS, Singh B. 2005. Rhodiola rosea: A versatile adaptogen. Comprehensive Reviews in Food Science and Food Safety. 4, 55-62.
- Leung KW, Wong KS. 2010. Pharmacology of ginsenosides: a literature review. Chinese Med. 5:20:1-7
- Kenakin TP. 2012. Pharmacology in drug discovery. Understanding drug response. Elsevier Inc. Amsterdam• Boston• Heidelberg• London• New York• Oxford. Paris • San Diego • San Francisco • Singapore • Sydney • Tokyo, 247 p.
- Pearce PT, Zois I, Wynne KN, et al. 1982. Panax ginseng and Eleuthrococcus senticosus extracts–in vitro studies on binding to steroid receptors. Endocrinol Jpn. 29: 567-573.
- Huo YS, Chen YZ, Yu ZY, et al. 1988. The effect of Panax ginseng extract (GS) on insulin and corticosteroid receptors. J Tradit Chin Med. 8:293-295
- Lee YJ, Chung E, Lee KY, et al. 1997. Ginsenoside-Rg1, one of the major active molecules from Panax ginseng, is a functional ligand of glucocorticoid receptor. Mol Cell Endocrinol. 133:135-140.
- Chung E, Lee KY, Lee YJ, et al. 1998. Ginsenoside Rg1 down-regulates glucocorticoid receptor and displays synergistic effects with cAMP. Steroids 63: 421-424.
- Lee YJ, Jin YR, Lim WC, et al. 2003. Ginsenoside-Rb1 acts as a weak phytoestrogen in MCF-7 human breast cancer cells. Arch Pharm Res. 26:58-63.
- Yan J, Liu Q, Dou Y, et al. 2013. Activating glucocorticoid receptor-ERK signaling pathway contributes to ginsenoside Rg1 protection against β-amyloid peptide-induced human endothelial cells apoptosis. J Ethnopharmacol.147:456-466.
- Song Y, Zhao F, Zhang L, et al. 2013. Ginsenoside Rg1 exerts synergistic anti-inflammatory effects with low doses of glucocorticoids in vitro. Fitoterapia. 91:173-179.
- Gao Y, Chu S, Li J, et al. 2015. Anti-inflammatory function of ginsenoside Rg1 on alcoholic hepatitis through glucocorticoid receptor related nuclear factor-kappa B pathway. J Ethnopharmacol. 173: 231-240.
- Sun XC, Ren XF, Chen L, et al. 2016. Glucocorticoid receptor is involved in the neuroprotective effect of ginsenoside Rg1 against inflammation-induced dopaminergic neuronal degeneration in substantia nigra. J Steroid Biochem Mol Biol. 155 (Pt A): 94-103.
- Nah SY. 2012. Gintonin: a novel ginseng-derived ligand that targets G protein- coupled lysophosphatidic acid receptors. Curr Drug Targets. 13:1659-1664.
- Lee YJ,.Cho JY, Kim JH, et al. 2004. Extracts from Schizandra chinensis fruit activate estrogen receptors: a possible clue to its effects on nitric oxide-mediated vasorelaxation. Biol Pharm Bull. 27:1066-1069.
- Cao X, Jiang J, Zhang S, et al. 2013. Discovery of natural estrogen receptor modulators with structure-based virtual screening. Bioorg Med Chem Lett. 23: 3329-3333.
- Kim MH, Choi YY, Han JM, et al.2014. Ameliorative effects of Schizandra chinensis on osteoporosis via activation of estrogen receptor (ER)-α/-β. Food Funct. 5:1594-1601.
- Gerbarg PL, Brown RP. 2016. Pause menopause with Rhodiola rosea, a natural selective estrogen receptor modulator. Phytomedicine. 23:763-769.
- Bassa LM, Jacobs C, Gregory K, et al. 2016. Rhodiola crenulata induces an early estrogenic response and reduces proliferation and tumorsphere formation over time in MCF7 breast cancer cells. Phytomedicine. 23:87-94.
- Hahm ER, Lee J, Huang Y, et al. 2011. Withaferin a suppresses estrogen receptor-α expression in human breast cancer cells. Mol Carcinog. 50: 614-624.
- Ravindran R, Sharma N, Roy S, et al. 2015. Interaction Studies of Withania somnifera’s Key Metabolite Withaferin A with Different Receptors Associated with Cardiovascular Disease. Curr Comput Aided Drug Des. 11: 212-221.
- Khazal KF, Hill DL, Grubbs CJ. 2014. Effect of Withania somnifera root extract on spontaneous estrogen receptor-negative mammary cancer in MMTV/Neu mice. Anticancer Res. 34: 6327-6332.
- Lau WS, Chan RY, Guo DA, et al. 2008. Ginsenoside Rg1 exerts estrogen-like activities via ligand-independent activation of ERalpha pathway. J Steroid Biochem Mol Biol. 108:64-71.
- Chan RY, Chen WF, Dong A, et al. 2002. Estrogen-like activity of ginsenoside Rg1 derived from Panax notoginseng. J Clin Endocrinol Metab. 87: 3691-3695.
- Cho J, Park W, Lee S, et al. 2004. Ginsenoside-Rb1 from Panax ginseng C.A. Meyer activates estrogen receptor-alpha and -beta, independent of ligand binding. J Clin Endocrinol Metab. 89:3510-3515.
- Olsson EMG, von Schele B, Panossian AG. A randomized double-blind placebo controlled parallel group study of SHR-5 extract of Rhodiola Rosea Roots as treatment for patients with stress related fatigue. Plant Medica. 2009 Feb; 75 (2): 105-12.
- Darbinyan V, Kteyan A, Panossian A, Gabrielian E, Wikman G, Wagner H. Rhodiola rosea in stress induced fatigue–a double blind cross-over study of a standardized extract SHR-5 with a repeated low-dose regimen on the mental performance of healthy physicians during night duty. Phytomedicine. 2000 Oct;7(5):365-71.
- Aslanyan G, Amroyan E, Gabrielyan E, Nylander M, Wikman G, Panossian A. 2010. “Double-blind, placebo-controlled, randomized study of single dose effects of Adapt -232 on cognitive functions “. Phytomedicine, 17, pp. 494-499.
- Bogatova, RI, Shlykova, LV, Salnitsky, VP, Wikman, G., 1997, “Evaluation of the effect of a single dose of a phytoadaptogen on the working capacity of human subjects during prolonged isolation.” Aerospace and Environmental Medicine. 31 , 4, pp. 51-54.
- Narimanian M, Badalyan M, Panosyan V, Gabrielyan E, Panossian A, Wikman G, Wagner H. 2005, “Impact of ChisanR (ADAPT 232) on the Quality – Of – Life and its Efficacy as an Adjuvant in the Treatment of Acute Non-Specific Pneumonia “, Phytomedicine. 12, pp 723-729.
- Panossian AG, Oganessian AS, Ambartsumian M, Gabrelian ES, Wagner H, Wikman G. 1999, Phytomedicine. 6, Effects of heavy physical exercise and adaptations on nitric oxide content in human saliva. , 1, pp. 17-26.
- Facchinetti F, Neri I, Tarbusi M. 2002. “Eleutherococcus senticocus reduces cardiovascular stress response in healthy subjects: randomized, placebo-controlled trial.” Stress Health. 18, pp. 11-17.
- Hovhannisyan AS, Panossian AG, Wikman G. 2015. ADAPT-232 and ADAPT-S for stress-induced fatigue and recovery of trained and elite athletes: a randomized, controlled trial. J Athl Enhancement. 4:4. http://dx.doi.org/10.4172/2324-9080.1000205
- Megna M, Amico AP, Cristella G, Saggini R, Jirillo E, Ranieri M. 2012. Effects of herbal supplements on the immune system in relation to exercise. Int J Immunopathol Pharmacol. 25(1 Suppl):43S-49S.
- Darbinyan V, Aslanyan G, Amroyan E, Gabrielyan E, Malmström C, Panossian A. 2007. Clinical trial of Rhodiola rosea L. extract SHR-5 in the treatment of mild to moderate depression. Nord J Psychiatry. 61(5):343-8. Erratum in: Nord J Psychiatry. 2007;61(6):503. PubMed PMID: 17990195.
- Mao JJ, Xie SX, Zee J, Soeller I, Li QS, Rockwell K, Amsterdam JD. 2015. Rhodiola rosea versus sertraline for major depressive disorder: A randomized placebo-controlled trial. Phytomedicine. 22(3):394-9.
- Panossian A, Amsterdam J. 2017. Adaptogens in Psychiatric Practice. Chapter 8 In: Complementary and Integrative Treatments in Psychiatric Practice. Rds. Gerbarg PL, Muskin PR, Brown RP, American Psychiatric Publishing. Pp.155-181.
- Sarris J, Panossian A, Schweitzer I, Stough C, Scholey A. 2011. Herbal medicine for depression, anxiety and insomnia: a review of psychopharmacology and clinical evidence. Eur Neuropsychopharmacol.21(12):841-60. https://www.sciencedirect.com/science/article/pii/S0924977X1100071X
- Estonian Ministry of Health Affairs, 1998. Regulation No. 7, Annex 1, 21st January, Government of Estonia, Tallin.
- National Pharmacopoeia of the USSR, 1990. 11th Edn – Pharmacopoeia paper 75, Rhizome and roots of Rhodiola rosea, The USSR Ministry of Health, Moscow, Meditsina, 2(1), 317-319; update No 2 dated 19-05-1999.
- Thakur AK, Chatterjee SS, Kumar V. 2014. Adaptogenic potential of andrographolide: An active principle of the king of bitters (Andrographis paniculata). J Tradit Complement Med. 5:42-50.
- Panossian A, Seo EJ, Efferth T. 2019. Effects of anti-inflammatory and adaptogenic herbal extracts on gene expression of eicosanoids signaling pathways in isolated brain cells. Phytomedicine. 2019 Mar 10:152881. https://www.sciencedirect.com/science/article/pii/S0944711319300510?via%3Dihub
- Panossian A, Seo EJ, Efferth T. 2018. Novel molecular mechanisms for the adaptogenic effects of herbal extracts on isolated brain cells using systems biology. Phytomedicine. 50:257-284. https://www.sciencedirect.com/science/article/pii/S0944711318304835?via%3Dihub
- Seo EJ, Klauck SM, Efferth T, Panossian A. 2019. Adaptogens in chemobrain (Part I): Plant extracts attenuate cancer chemotherapy-induced cognitive impairment – Transcriptome-wide microarray profiles of neuroglia cells. Phytomedicine.55:80-91. https://www.sciencedirect.com/science/article/pii/S0944711318305439?via%3Dihub
- Seo EJ, Klauck SM, Efferth T, Panossian A. 2018. Adaptogens in chemobrain (Part II): Effect of plant extracts on chemotherapy-induced cytotoxicity in neuroglia cells. Phytomedicine. 58:152743. https://www.sciencedirect.com/science/article/pii/S0944711318305610?via%3Dihub
- Seo EJ, Klauck SM, Efferth T, Panossian A. 2019. Adaptogens in chemobrain (Part III): Antitoxic effects of plant extracts towards cancer chemotherapy-induced toxicity – transcriptome-wide microarray analysis of neuroglia cells. Phytomedicine. 56:246-260. https://www.sciencedirect.com/science/article/pii/S0944711318305683?via%3Dihub
- Panossian A, Seo EJ, Klauck SM, Efferth T. Adaptogens in chemobrain (part IV): adaptogenic plants prevent the chemotherapeutics-induced imbalance of redox homeostasis by modulation of expression of genes encoding Nrf2-mediated signaling proteins and antioxidant, metabolizing, detoxifying enzymes in neuroglia cells.Longhua Chin Med 2020. ;3:4 doi: 10.21037/lcm-20-24. http://lcm.amegroups.com/article/view/6379
- Panossian A, Seo EJ, Efferth T. Synergy assessments of plant extracts used in the treatment of stress and aging-related disorders, Synergy Research 7 (2018) 39-49.
https://doi.org/10.1016/j.synres.2018.10.001
https://www.sciencedirect.com/science/article/pii/S2213713018300312?via%3Dihub - Narimanyan M, Jamalyan K. Balyan A, Barth A, Palm S, Wikman G, Panossian A. Early intervention with Kan Jang® to treat upper-respiratory-tract infections: A randomized, quadruple-blind study, Journal of Traditional and Complementary Medicine, 2021, https://doi.org/10.1016/j.jtcme.2021.06.001
- Pkhaladze L, Davidova N, Khomasuridze A, Shengelia R, Panossian AG. Actaea racemosa L. Is More Effective in Combination with Rhodiola rosea L. for Relief of Menopausal Symptoms: A Randomized, Double-Blind, Placebo-Controlled Study. Pharmaceuticals (Basel). 2020 May 21;13(5):102. doi: 10.3390/ph13050102.
- Dimpfel W, Schombert L, Keplinger-Dimpfel IK, Panossian A. Effects of an Adaptogenic Extract on Electrical Activity of the Brain in Elderly Subjects with Mild Cognitive Impairment: A Randomized, Double-Blind, Placebo-Controlled, Two-Armed Cross-Over Study. Pharmaceuticals (Basel). 2020 Mar 14;13(3). pii: E45. doi: 10.3390/ph13030045. https://www.mdpi.com/1424-8247/13/3/45/htm
- Mariage PA, Hovhannisyan A, Panossian AG. Efficacy of Panax ginseng Meyer Herbal Preparation HRG80 in Preventing and Mitigating Stress-Induced Failure of Cognitive Functions in Healthy Subjects: A Pilot, Randomized, Double-Blind, Placebo-Controlled Crossover Trial. Pharmaceuticals (Basel). 2020 Mar 29;13(4).pii: E57. doi: 10.3390/ph13040057. https://www.mdpi.com/1424-8247/13/4/57/htm
- Lemerond T, Panossian AG (2020) Panax ginseng Meyer Herbal Preparation HRG80 for Preventing and Mitigating Stress-Induced Failure of Cognitive Functions in Healthy Subjects. J Altern Complement Integr Med 6: 100.
- Panossian AG, Efferth T, Shikov AN Pozharitskaya ON, Kuchta K, Mukherjee PK, Banerjee S, Heinrich M, Wu W, Guo D, Wagner H. Evolution of the adaptogenic concept from traditional use to medical systems: Pharmacology of stress‐ and aging‐related diseases. Med Res Rev. 2021; 41 (1). 630-703. https://doi.org/10.1002/med.21743; https://onlinelibrary.wiley.com/doi/10.1002/med.21743; http://media.phytomed.se/2020/12/Video_Dec_25_2020.mp4
- Panossian A, Brendler T. The Role of Adaptogens in Prophylaxis and Treatment of Viral Respiratory Infections. Pharmaceuticals (Basel). 2020 Sep 8;13(9): E236. doi: 10.3390/ph13090236. PMID:32911682.
- Brendler T, Al-Harrasi A, Bauer R, Gafner S, Hardy ML, Heinrich M, Hosseinzadeh H, Izzo AA, Michaelis M, Nassiri-Asl M, Panossian A, Wasser SP, Williamson EM. Botanical drugs and supplements affecting the immune response in the time of COVID-19: Implications for research and clinical practice. Phytotherapy Research. 2020;1–19. https://doi.org/10.1002/ptr.7008.
- Dimpfel W, Mariage PA, Panossian AG. Effects of Red and White Ginseng Preparations on Electrical Activity of the Brain in Elderly Subjects: A Randomized, Double‐Blind, Placebo‐Controlled, Three‐Armed Cross‐Over Study. Pharmaceuticals 2021, 14, 182. https://doi.org/10.3390/ph14030182
Panossian, A.; Abdelfatah,S.; Efferth, T. Network Pharmacology of Red Ginseng (Part I): Effects of Ginsenoside Rg5 at Physiological and Sub-Physiological Concentrations. Pharmaceuticals 2021, 14, 999. . https://doi.org/10.3390/ph14100999
Panossian, A.; Abdelfatah,S.; Efferth, T. Network Pharmacology of Ginseng (Part II): The Differential Effects of Red Ginseng and Ginsenoside Rg5 in Cancer and Heart Diseases as Determined by Transcriptomics. Pharmaceuticals 2021, 14(10), 1010; https://doi.org/10.3390/ph14101010 .
- Panossian, A., Abdelfatah, S., & Efferth, T. (2022). Network Pharmacology of Ginseng (Part III): Antitumor Potential of a Fixed Combination of Red Ginseng and Red Sage as Determined by Transcriptomics. Pharmaceuticals (Basel, Switzerland), 15(11), 1345. https://doi.org/10.3390/ph15111345
- Panossian, A., & Efferth, T. (2022). Network Pharmacology of Adaptogens in the Assessment of Their Pleiotropic Therapeutic Activity. Pharmaceuticals (Basel, Switzerland), 15(9), 1051. https://doi.org/10.3390/ph15091051
- Ratiani, L., Pachkoria, E., Mamageishvili, N., Shengelia, R., Hovhannisyan, A., & Panossian, A. (2022). Efficacy of Kan Jang® in Patients with Mild COVID-19: Interim Analysis of a Randomized, Quadruple-Blind, Placebo-Controlled Trial. Pharmaceuticals (Basel, Switzerland), 15(8), 1013. https://doi.org/10.3390/ph15081013
- Karosanidze I, Kiladze U, Kirtadze N, Giorgadze M, Amashukeli N, Parulava N, Iluridze N, Kikabidze N, Gudavadze N, Gelashvili L, Koberidze V, Gigashvili E, Jajanidze N, Latsabidze N, Mamageishvili N, Shengelia R, Hovhannisyan A, and Panossian A. Efficacy of Adaptogens in Patients with Long COVID-19: A Randomized, Quadruple-Blind, Placebo-Controlled Trial. Pharmaceuticals 2022, 15(3), 345; https://doi.org/10.3390/ph15030345
- Panossian, A.Herba Sideritis: A putative adaptogen for reducing the risk of age-related cognitive decline and neurodegenerative disorders Phytomedicine Plus, Volume 4, Issue 1,2024,100519; https://doi.org/10.1016/j.phyplu.2023.100519; https://www.sciencedirect.com/science/article/pii/S266703132300115X
- Panossian A, Lemerond T.
Herba Sideritis: Insights from a Herbal Monograph with Particular Reference to Adaptogenic Potential in Neurodegenerative Disorders and Aging-related Cognitive Decline, Edition 1, BP International 2024; October 2024, Page 1-58. ISBN 978-93-48119-26-1 (Print) ISBN 978-93-48119-56-8 (eBook) DOI: https://doi.org/10.9734/bpi/mono/978-93-48119-26-1;
Panossian, A., Lemerond, T., & Efferth, T. (2024). State-of-the-Art Review on Botanical Hybrid Preparations in Phytomedicine and Phytotherapy Research: Background and Perspectives. Pharmaceuticals (Basel, Switzerland), 17(4), 483. https://doi.org/10.3390/ph17040483
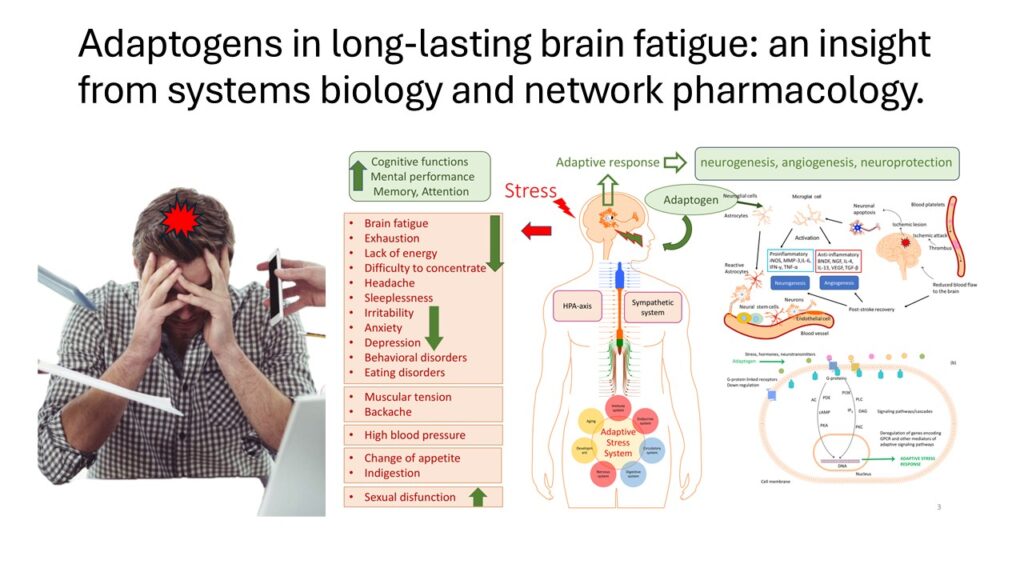
- Panossian, A., Lemerond, T., & Efferth, T. (2025). Adaptogens in Long-Lasting Brain Fatigue: An Insight from
Systems Biology and Network Pharmacology. Pharmaceuticals (Basel, Switzerland), 118, 261. https://doi.org/10.3390/
ph18020261; https://www.mdpi.com/1424-8247/18/2/261
